
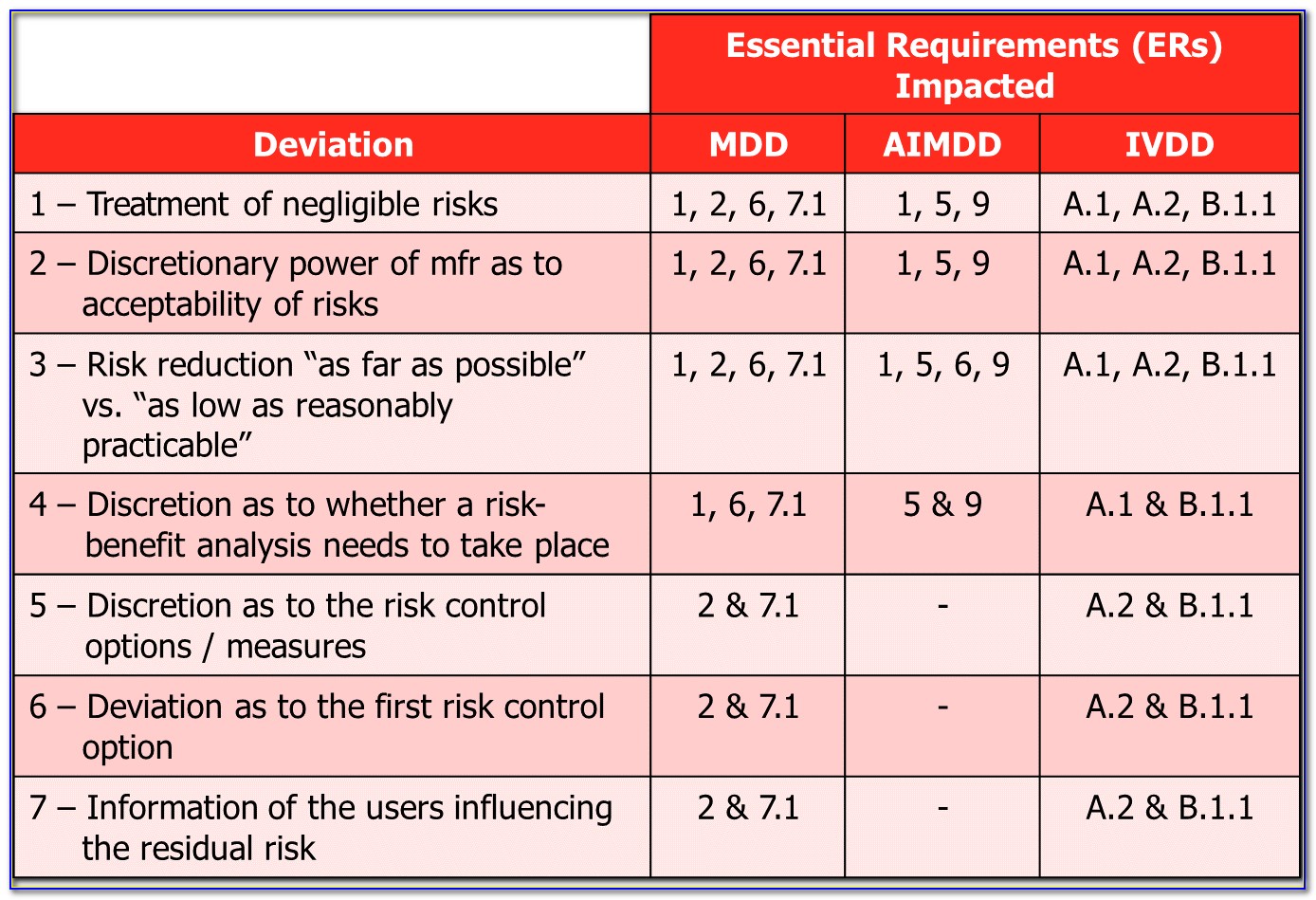
Do you know what the other three are? The second type is process risk management where you document your risk estimation in a process risk analysis. The one most people are familiar with is risk analysis associated with the design of a medical device. Instead, this article is describing the four different q uality system processes that need risk analysis. FMEA, Fault Tree Analysis, HAZOP, HACCP, etc.). Most people immediately think that this is going to be a tutorial about four different tools for risk management (e.g. It means there are different levels of risks associated with a device and we should estimate each risk for its acceptability to use on humans.Last week’s YouTube live streaming video answered the question, “What are the four different types of risk analysis?” Everyone in the medical device industry is familiar with ISO 14971:2019 as the standard for medical device risk management, but most of us are only familiar with two or three ways to analyze risks. There are different levels of severities for different types of harms for a particular medical device, based on its intended use.
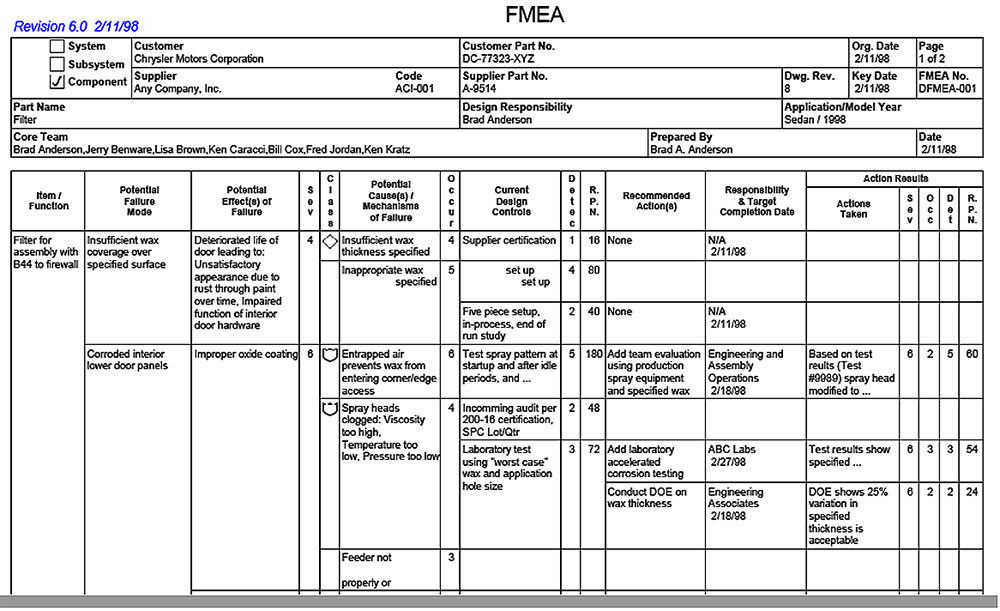
If we came to know that there is a probability of occurrence of harm, associated with the use of particular medicine or device, then we should estimate the severity of that harm. Similar way, the products which we are used for the treatment of patients having some side effects or harms, but we should use them to save the lives. There is a saying, “A bitter pill to swallow”, which means that we have to accept it even though it is very unpleasant. So, we should compare the benefits with the risks, to determine the acceptability of risks of a particular medical device. Medical devices are developed for certain medical purposes, means they have certain benefits. Risk Benefit Analysis of Medical Devices is tough and interesting.


 0 kommentar(er)
0 kommentar(er)
